PRECLINICAL DMPK APPLICATIONS
NORMOTHERMIC PERFUSED PORCINE ORGANS
CONTRACT SERVICE, TECHNOLOGIES OPEN FOR PARTNERING
Ex vivo whole organ normothermic machine perfusion model
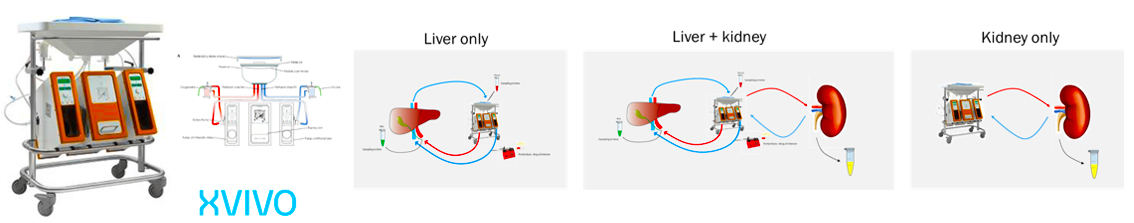
TNO has developed normothermic whole organ perfusion model as a new tool to predict preclinical ADME. Over the last couple of years major developments have been achieved in the field of organ transplantation.
Various machines have been developed that are used in transplantation research to monitor organ viability prior to transplantation. One of the developed machines is the Liver Assist, a pressure driven perfusion machine with continuous portal flow and pulsatile arterial flow.
We apply porcine organs as a good substitute for human organs.
Advantages of using Normothermic Whole Organ Perfusion model for drug research:
● Mimicking of physiological conditions: It is one of the effective options to preserve and study organs under physiological conditions. By maintaining physiological conditions, including blood flow, bile and urine production and keeping the organs close to body temperature, it is possible to more faithfully mimic the reactions and changes that occur within the body.
● Long-term preservation: Extended organ preservation time enables in-depth research into drug efficacy and safety.
● Elucidation of pharmacokinetics: Pharmacokinetics and metabolism of drugs can be evaluated under more realistic conditions. It is possible to predict clinical pharmacokinetics and metabolic profile, the potential impact of Drug-Drug Interactions on these processes and how fast and in what way the drug will be cleared.
● Research on complex diseases: It may be possible to examine the effects of drugs on complex diseases and disorders in more detail. For example: the possibility of collecting data to develop treatments for fibrosis, inflammation and damage in specific organs.
These advantages make the normothermic whole organ perfusion model extremely useful in pharmaceutical research and contribute to the development of new treatments and therapeutic strategies in clinical applications.
Ex vivo Liver perfusion model
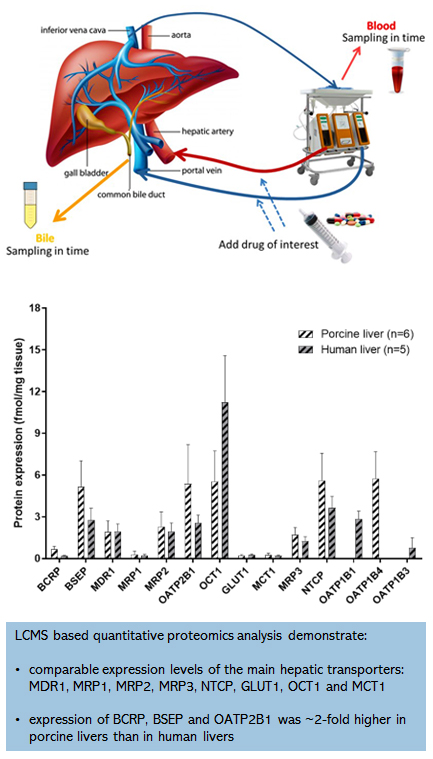 In fact porcine livers are a good substitute for human livers can be shown by looking at the absolute expression levels of transporters and metabolic enzymes.
For this we used LCMS based quantitative proteomic analysis and found comparable expression levels of the main hepatic transporters: MDR1, MRP1, MRP2, MRP3, NTCP, GLUT1, OCT1 and MCT1.
Only the expression of the transporters BCRP, BSEP and OATP2B1 was ~2-fold higher in porcine livers than in human livers. Our findings were confirmed in a recent study by Elmorsi et al,
in which they also compared human and porcine liver proteomics, and also found that the main hepatic CYP and UGT enzymes share high similarity and comparable expression levels between porcine and human livers.
In fact porcine livers are a good substitute for human livers can be shown by looking at the absolute expression levels of transporters and metabolic enzymes.
For this we used LCMS based quantitative proteomic analysis and found comparable expression levels of the main hepatic transporters: MDR1, MRP1, MRP2, MRP3, NTCP, GLUT1, OCT1 and MCT1.
Only the expression of the transporters BCRP, BSEP and OATP2B1 was ~2-fold higher in porcine livers than in human livers. Our findings were confirmed in a recent study by Elmorsi et al,
in which they also compared human and porcine liver proteomics, and also found that the main hepatic CYP and UGT enzymes share high similarity and comparable expression levels between porcine and human livers.
Application:
● Porcine Liver model
> Hepatic Clearance
> Biliary Excretion
> Drug-drug interactions
> Mechanistic study of transporter involvement in hepatic drug clearance
> Metabolite formation, Metabolite production
> De Novo Lipogenesis
● Human Liver model
> Hepatic Clearance
> Hepatic Metabolism
> Biliary Excretion
> Plasma Exposure upon DDI
> Dosing to portal vein and/or hepatic artery
> NASH: Biomarker detection / Target Binding
Download, Publication link:
●
Novel Explanted Human Liver Model to Assess Hepatic Extraction, Biliary Excretion and Transporter Function (Clinical Pharmacology and Therapeutics, April 2023)
●
Evaluation of Normothermic Machine Perfusion of Porcine Livers as a Novel Preclinical Model to Predict Biliary Clearance and Transporter-mediated Drug-Drug Interactions using Statins (Drug Metabolism and Disposition, July 2021)
●
HepMetPro; - Hepatic Metabolite Production -
●
De Novo Lipogenesis using 14C-labeled acetate and Accelerator Mass Spectrometry
●
Normothermic Machine Perfusion of Diseased Explanted Livers to Study Hepatic Pharmacokinetic Processes (Poster at ISSX-2021)
●
Ex vivo whole liver perfusion model for prediction of drug drug interactions and biliary excretion of Rosuvastatin (Poster)
●
Pharmacokinetics Application of Normothermic Perfused Ex Vivo Porcine Livers. Poster ISSX-2019
Ex vivo Kidney perfusion model
 Application: TO STUDY RENAL CLEARANCE OF DRUGS
Application: TO STUDY RENAL CLEARANCE OF DRUGS
● Experimental set up;
> Infusion of drug cocktail to study renal clearance of probe drug cocktail (0 - 120 min)
> Study DDI at the renal level by infusing probe + inhibitor cocktail mix (125- 245 min)
> Perfusate and urine drug profiles
● Measurement;
> Flow (pressure driven machine)
> Inulin clearance
> Urine production
> Ad hoc viability analysis
> Perfusate: pO2, pCO2, pH, sO2, ureum,creatinin, Na, K
> Albumin (in perfusate and urine)
> Biopsy: before, during & end of perfusion
Combined Liver and Kidney perfusion model
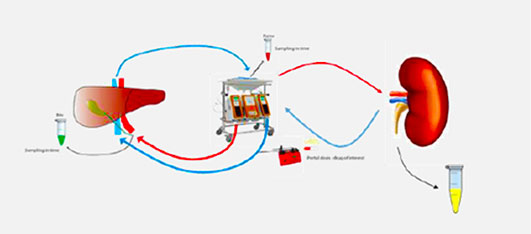 Application: To study Clearance of Probe Drug Cocktail via Biliary and Renal Clearance route
Application: To study Clearance of Probe Drug Cocktail via Biliary and Renal Clearance route
● Determine % of biliary and % renal excretion of drugs
● Ability to perfuse for longer time
● Open for Partnering
Download:
□
Optimized Normothermic Machine Perfusion of Liver and Kidney used to Predict Human Pharmacokinetics. (Poster at JSSX, 2023)
□
Combined Ex Vivo Liver and Kidney Perfusion to Predict Biliary and Renal Clearance.
Predicting Human Pharmacokinetics by using an ex vivo new multi-organ perfusion model
To demonstrate and incorporate ADME data of 2 model drugs (rosuvastatin and digoxin) derived from intestinal segment studies and whole organ perfused liver and kidney into a generic PBPK model to predict the drug PK profile in humans.
Publication Download:
□ Demonstrator study using Rosuvastatin and Digoxin.
□ Ex vivo gut-hepato-biliary organ perfusion model to characterize oral absorption, gut-wall metabolism, pre-systemic hepatic metabolism and biliary excretion; application to midazolam


